
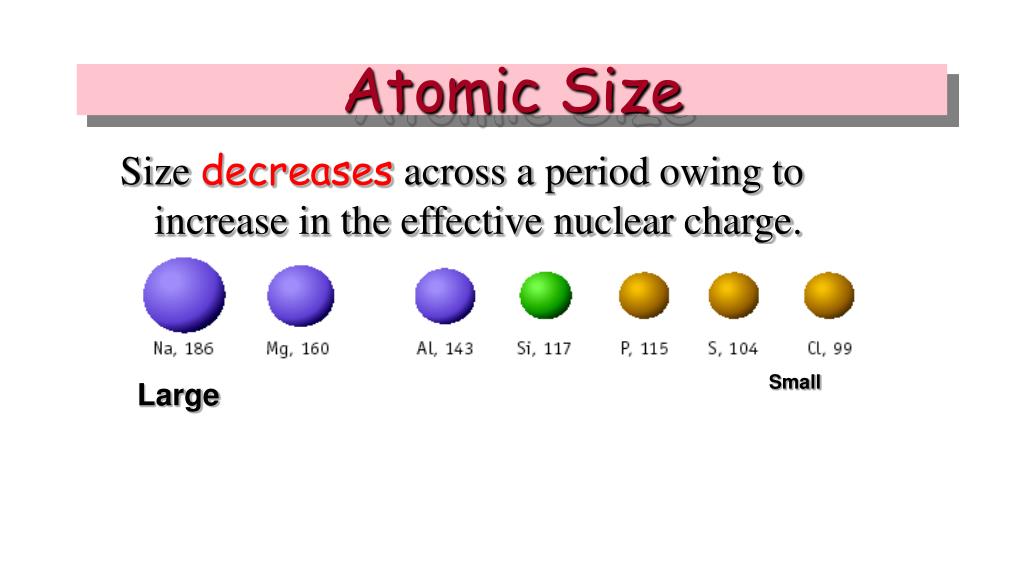
This is an example of how these elements behave differently, and a reminder that trends do not necessarily apply to every instance. The size of an atom generally decreases as one moves from left to right for a certain period. Two elements decrease and then increase again, seemingly throwing the trend off. On the bottom of the table we see a slight bump in the trend.
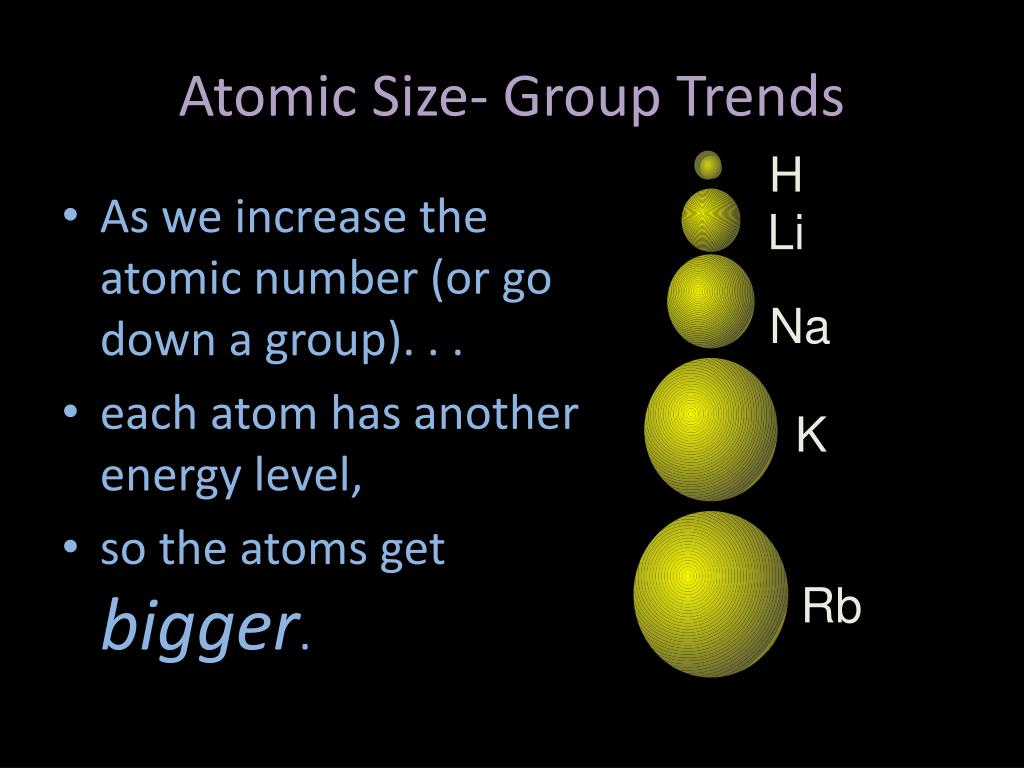
When an electron is added, a new proton is also added to the nucleus, which. Within a period of elements, each new electron is added to the same shell. The first atomic radius periodic trend is that atomic size decreases as you move left to right across a period. However as we go down the group the situation of the equation is reversed and so our atoms become larger as their core charge gets smaller. Atomic Radius Trend 1: Atomic Radii Decrease From Left to Right Across a Period. This equation fits perfectly with the above diagram and explains why the atoms get smaller as they travel across the period. As this number gets higher, the valence electrons are pulled closer to the nucleus, therefore decreasing the atomic size of the atom. The core charge is simply an expression of the attractive force that the centre of the nucleus gives off to the valence electrons.

CORE CHARGE = PROTONS - NON-VALENCE ELECTRONS. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period.Ītomic radius can be linked to core charge. With the above image, courtesy of Webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table.Ītomic radius is measured from the centre of the nucleus to the outermost electron shell.


 0 kommentar(er)
0 kommentar(er)
